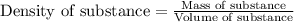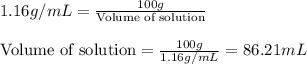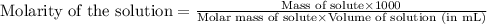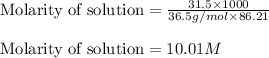Chemistry, 22.07.2019 05:00 genesisheaven1
Muriatic acid is an old name for hydrochloric acid. the commercial grade (impure) solution is still sold as muriatic acid. you use it in toilet bowl cleaners, for cleaning masonry, and for adjusting the ph of swimming pools. my local hardware store sells muriatic acid labelled as 31.5 % hcl. its density is 1.16 g/ml. assume you have 1 l of this muriatic acid (ma). determine the molarity given this information.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 06:00, coolkid2041
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
Muriatic acid is an old name for hydrochloric acid. the commercial grade (impure) solution is still...
Questions in other subjects:


Mathematics, 30.08.2020 01:01


Mathematics, 30.08.2020 01:01

Arts, 30.08.2020 01:01


English, 30.08.2020 01:01