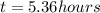How long must a constant current of 50.0 a be passed through an electrolytic cell containing aqueous cu2+ ions to produce 5.00 moles of copper metal? how long must a constant current of 50.0 a be passed through an electrolytic cell containing aqueous cu2+ ions to produce 5.00 moles of copper metal? 5.36 hours 2.68 hours 0.373 hours 0.187 hours?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 07:30, superfly903
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2
You know the right answer?
How long must a constant current of 50.0 a be passed through an electrolytic cell containing aqueous...
Questions in other subjects:


Mathematics, 15.10.2019 05:00

Biology, 15.10.2019 05:00


History, 15.10.2019 05:00

Mathematics, 15.10.2019 05:00




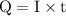
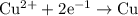
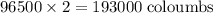
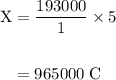
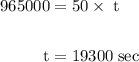
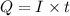
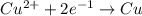
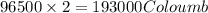 of electricity deposits 1 mole of Cu.
of electricity deposits 1 mole of Cu.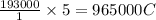
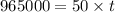
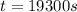 (1 hour=3600sec)
(1 hour=3600sec)