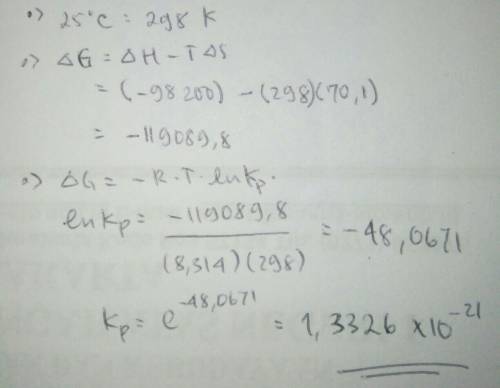Chemistry, 23.07.2019 04:00 kdndiamond9101
Hydrogen peroxide (h2o2) decomposes according to the equation: h2o2(l) ⇆ h2o(l) + ½ o2(g)calculate kp for this reaction at 25°c. (δh° = –98.2 kj/mol, δs° = 70.1 j/k·mol)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 03:00, Cheyenne7327
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1
You know the right answer?
Hydrogen peroxide (h2o2) decomposes according to the equation: h2o2(l) ⇆ h2o(l) + ½ o2(g)calculate k...
Questions in other subjects:

Mathematics, 25.03.2021 02:00



Mathematics, 25.03.2021 02:00

Biology, 25.03.2021 02:00

Mathematics, 25.03.2021 02:00

Mathematics, 25.03.2021 02:00