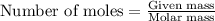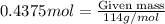Carbon dioxide is a green house gas that is linked to global warming. it is released into the atmosphere through the combustion of octane (c8h18) in gasoline. write the balanced chemical equation for the combustion of octane. (use the lowest possible whole number coefficients. omit states-of-matter from your answer.)
calculate the mass of octane needed to release 3.50 mol co2.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, girlchamp654
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2
You know the right answer?
Carbon dioxide is a green house gas that is linked to global warming. it is released into the atmosp...
Questions in other subjects:




Mathematics, 15.12.2021 16:30

Mathematics, 15.12.2021 16:30

Social Studies, 15.12.2021 16:30



History, 15.12.2021 16:30


 of octane.
of octane.