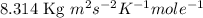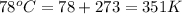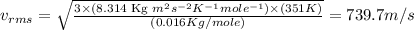Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:00, bakoeboo
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2
You know the right answer?
Calculate the root-mean-square speed of methane, ch4 (g), at 78°c. root mean square speed = u = [3rt...
Questions in other subjects:

Mathematics, 13.09.2020 05:01

Mathematics, 13.09.2020 05:01

Biology, 13.09.2020 05:01

Mathematics, 13.09.2020 05:01

Mathematics, 13.09.2020 05:01

Mathematics, 13.09.2020 05:01

Mathematics, 13.09.2020 05:01

Mathematics, 13.09.2020 05:01

Mathematics, 13.09.2020 05:01

Mathematics, 13.09.2020 05:01

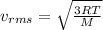
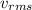 = root mean square speed
= root mean square speed