

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2


Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 01:00, skatelife8974
What is the chemical name of the compound ti2o3?
Answers: 2
You know the right answer?
A0.100 m solution of a monoprotic weak acid has a ph of 3.00. what is the pka of this acid?...
Questions in other subjects:





Mathematics, 25.02.2020 01:58



Mathematics, 25.02.2020 01:58

Social Studies, 25.02.2020 01:58

![-log [H^{+}]](/tpl/images/0123/3207/822be.png)
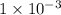
![[H^{+}] = 1 \times 10^{-3}](/tpl/images/0123/3207/7e0f1.png) M
M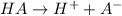
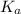 ,
, ![[H^{+}]](/tpl/images/0123/3207/85507.png) and HA is as follows.
and HA is as follows.![K_{a} = \frac{[H^{+}][A^{-}]}{[HA]}](/tpl/images/0123/3207/04688.png)
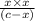
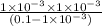
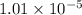
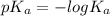
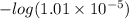
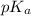 of given acid is 5.0.
of given acid is 5.0.


