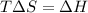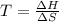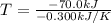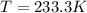Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 01:00, daniel1480
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
If δh = -70.0 kj and δs = -0.300 kj/k , the reaction is spontaneous below a certain temperature. cal...
Questions in other subjects:


Mathematics, 06.09.2020 03:01



Mathematics, 06.09.2020 03:01



History, 06.09.2020 03:01

Biology, 06.09.2020 03:01

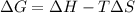
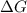 = Gibbs free energy
= Gibbs free energy 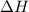 = enthalpy change = -70 kJ
= enthalpy change = -70 kJ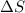 = entropy change = -0.300 kJ/K
= entropy change = -0.300 kJ/K