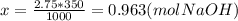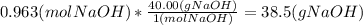Chemistry, 23.07.2019 16:00 bustillojoshua4
Zach needs to make a 2.75 m solution of naoh(molar mass=40.00g/mol) using 350. g of water. what mass of naoh should he dissolve in the water to make this solution? show the steps

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, alydiale584
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1


Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
Zach needs to make a 2.75 m solution of naoh(molar mass=40.00g/mol) using 350. g of water. what mass...
Questions in other subjects:




Mathematics, 23.04.2021 01:10

Business, 23.04.2021 01:10

History, 23.04.2021 01:20


Mathematics, 23.04.2021 01:20


 ,
,