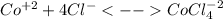In an aqueous chloride solution cobalt(ii) exists in equilibrium with the complex ion cocl42-. co2+(aq) is pink and cocl42-(aq) is blue. at low temperature the pink color predominates. at high temperature the blue color is strong. if we represent the equilibrium as: +(aq) + 4cl-(aq) cocl42-(aq) we can conclude that: 1. this reaction is: a. exothermic b. endothermic c. neutral d. more information is needed to answer this question.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, johnnydenali67
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 01:00, jescanarias22
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
In an aqueous chloride solution cobalt(ii) exists in equilibrium with the complex ion cocl42-. co2+(...
Questions in other subjects:

Mathematics, 18.11.2019 16:31




Mathematics, 18.11.2019 16:31




Mathematics, 18.11.2019 16:31