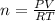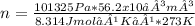Chemistry, 23.07.2019 17:00 elliedeegan3910
Asample of argon gas at stp occupies 56.2 liters. determine the number of moles of argon and the mass in the sample. for the above problem how will you rearrange the ideal gas law to solve for moles of argon?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 23.06.2019 01:30, elijahbebeastin
What are several ways to reduce the effect of friction
Answers: 2
You know the right answer?
Asample of argon gas at stp occupies 56.2 liters. determine the number of moles of argon and the mas...
Questions in other subjects:

Mathematics, 27.09.2019 11:30



Mathematics, 27.09.2019 11:30


Mathematics, 27.09.2019 11:30

Social Studies, 27.09.2019 11:30



Mathematics, 27.09.2019 11:30