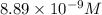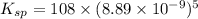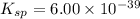Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, ander67061
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 19:00, Farhan54019
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 23.06.2019 01:00, davelopez979
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
The molar solubility of ba3(po4)2 is 8.89 x 10-9 m in pure water. calculate the ksp for ba3(po4)2. t...
Questions in other subjects:

Mathematics, 30.03.2020 03:52



History, 30.03.2020 03:52


Mathematics, 30.03.2020 03:52


Mathematics, 30.03.2020 03:52

Biology, 30.03.2020 03:52

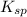 is
is 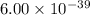
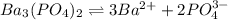
![K_{sp}=[Ba^{2+}]^3[PO_4^{3-}]^2](/tpl/images/0124/1385/f39cc.png)
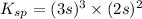
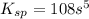
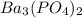 = s =
= s =