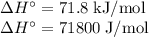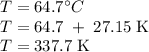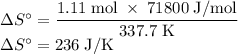Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, celestemaria0727
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 23.06.2019 03:30, alvfran1041
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1

Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 04:31, laurenbreellamerritt
How big are the bighest ocean waves at mavericks
Answers: 1
You know the right answer?
The normal boiling point of methanol is 64.7 °c and the molar enthalpy of vaporization if 71.8 kj/mo...
Questions in other subjects:


History, 31.10.2019 03:31

Spanish, 31.10.2019 03:31


Mathematics, 31.10.2019 03:31


Computers and Technology, 31.10.2019 03:31

Mathematics, 31.10.2019 03:31


Mathematics, 31.10.2019 03:31

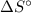 ) has been given as:
) has been given as: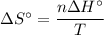
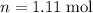
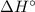 ),
),