Chemistry, 23.07.2019 21:00 janelisse199820
The combustion of gasoline produces carbon dioxide and water. assume gasoline to be pure octane (c8h18) and calculate the mass (in kg) of carbon dioxide that is added to the atmosphere per 1.3 kg of octane burned. (hint: begin by writing a balanced equation for the combustion reaction.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, tntaylor862
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1



Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
The combustion of gasoline produces carbon dioxide and water. assume gasoline to be pure octane (c8h...
Questions in other subjects:


History, 30.06.2021 01:40

Mathematics, 30.06.2021 01:40

Mathematics, 30.06.2021 01:40

Mathematics, 30.06.2021 01:40

Biology, 30.06.2021 01:40




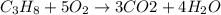
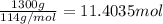
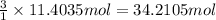 of carbon dioxide
of carbon dioxide