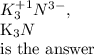Chemistry, 24.07.2019 02:30 cristabean87
What is the correct formula for the compound formed between potassium and nitrogen?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, hannah5143
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 21.06.2019 17:30, sophiebeardsley94
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

You know the right answer?
What is the correct formula for the compound formed between potassium and nitrogen?...
Questions in other subjects:


Mathematics, 21.07.2019 04:00




Chemistry, 21.07.2019 04:00

Mathematics, 21.07.2019 04:00



Physics, 21.07.2019 04:00