Chemistry, 24.07.2019 06:00 lilque6112
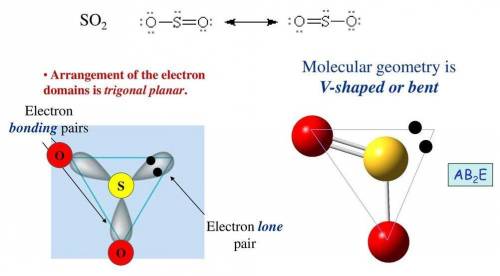
Etermine the electron geometry (eg), molecular geometry (mg), and polarity of so2. determine the electron geometry (eg), molecular geometry (mg), and polarity of . eg = tetrahedral, mg = tetrahedral, nonpolar eg = trigonal pyramidal, mg = trigonal pyramidal, polar eg = linear, mg = bent, nonpolar eg = tetrahedral, mg = linear, nonpolar eg = trigonal planar, mg = bent, polar

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1


Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
Etermine the electron geometry (eg), molecular geometry (mg), and polarity of so2. determine the ele...
Questions in other subjects:


Mathematics, 18.03.2021 02:00




Physics, 18.03.2021 02:00

English, 18.03.2021 02:00



Mathematics, 18.03.2021 02:00