
Chemistry, 24.07.2019 11:30 AshlynPlayz45
In 1909 fritz haber discovered the workable conditions under which nitrogen, n2(g), and hydrogen, h2(g), would combine using to produce ammonia. the conditions included medium temperature (~500oc), very high pressure (~351kpa), and an iron catalyst. the reaction is represented by the equation: n2(g) + 3h2(g) → 2nh3(g) how many grams of nitrogen are needed to produce 100 grams of ammonia gas?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, melidacampos12
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 23.06.2019 00:30, danielmartinez024m
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
In 1909 fritz haber discovered the workable conditions under which nitrogen, n2(g), and hydrogen, h2...
Questions in other subjects:


Mathematics, 18.05.2021 02:20


Chemistry, 18.05.2021 02:20

History, 18.05.2021 02:20


Mathematics, 18.05.2021 02:20


Physics, 18.05.2021 02:20

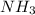 = 100 g
= 100 g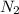 = 28 g/mole
= 28 g/mole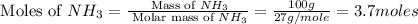
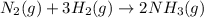
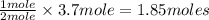 of
of 

