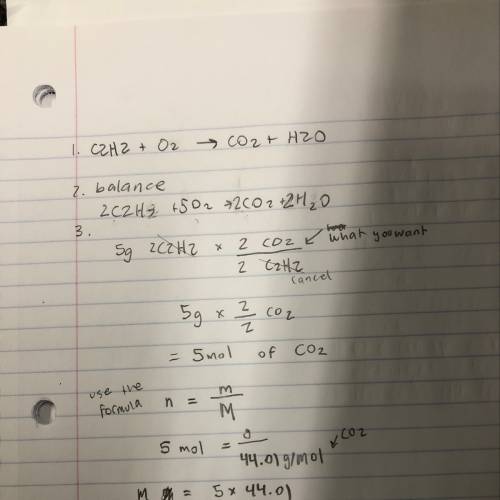Chemistry, 24.07.2019 16:00 tbenjamin1299
If the tank attached to a torch contains 5g of c2h2, how many grams of carbon dioxide will be produced

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:40, Islandgirl67
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b. zinc7.14,c. copper 8.92,d. lead 11.34
Answers: 2

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 16:00, annsmith66
What statement goes against the kinetic theory of gases
Answers: 1

Chemistry, 23.06.2019 00:40, joe7977
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
If the tank attached to a torch contains 5g of c2h2, how many grams of carbon dioxide will be produc...
Questions in other subjects:

Mathematics, 11.02.2020 05:05

Social Studies, 11.02.2020 05:05



Biology, 11.02.2020 05:05



Mathematics, 11.02.2020 05:05

Chemistry, 11.02.2020 05:05