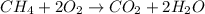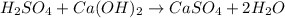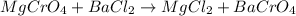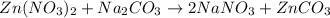Chemistry, 25.07.2019 06:30 Brittany0512
Which equation represents an oxidation-reduction reaction? 1.  ch4 + 2o2 → co2 + 2h2o 2.  h2so4 + ca(oh)2 → caso4 + 2h2o 3.  mgcro4 + bacl2 → mgcl2 + bacro4 4.  zn(no3)2 + na2co3 → 2nano3 + znco3?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:10, irene4523
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 07:20, camillexv2668
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

You know the right answer?
Which equation represents an oxidation-reduction reaction? 1.  ch4 + 2o2 → co2 + 2h2o 2.  h2so4 +...
Questions in other subjects:






History, 27.07.2019 02:30