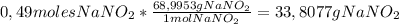Chemistry, 25.07.2019 06:30 raishagibson
A1.00 l solution contains 15.52 g of nitrous acid, hno2. what mass of sodium nitrite, nano2, should be added to it to make a buffer with a ph of 3.56? ka (hno2) = 4.0 × 10–4.

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
A1.00 l solution contains 15.52 g of nitrous acid, hno2. what mass of sodium nitrite, nano2, should...
Questions in other subjects:

Mathematics, 31.12.2019 17:31



Health, 31.12.2019 17:31




Mathematics, 31.12.2019 17:31


History, 31.12.2019 17:31