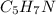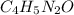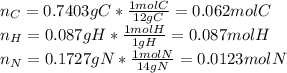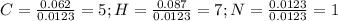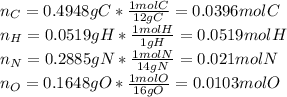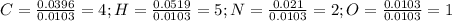Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, kingsqueen883
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3


Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
Calculate the empirical formula for each stimulant based on its elemental mass percent composition....
Questions in other subjects:

Biology, 29.03.2021 16:10





Mathematics, 29.03.2021 16:10



Computers and Technology, 29.03.2021 16:10

Chemistry, 29.03.2021 16:10