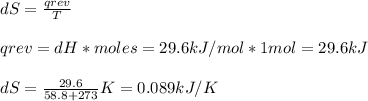Chemistry, 25.07.2019 11:00 kornut7316
The normal boiling point of bromine, br2(l), is 58.8°c, and its molar enthalpy of vaporization is δhvap = 29.6 kj/mol. (a) when br2(l) boils at its normal boiling point, does its entropy increase or decrease? decrease (δs is negative) increase (δs is positive) (b) calculate the value of δs when 1.00 mol of br2(l) is vaporized at 58.8°c.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, Playboycxm
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
The normal boiling point of bromine, br2(l), is 58.8°c, and its molar enthalpy of vaporization is δh...
Questions in other subjects:


Social Studies, 30.04.2021 04:30

Social Studies, 30.04.2021 04:30




Mathematics, 30.04.2021 04:30


English, 30.04.2021 04:30

Mathematics, 30.04.2021 04:30