
Chemistry, 20.08.2019 11:00 floressavanna15
4. calculate the molecular mass of:
a. hf
b. ni3
c. p4o10
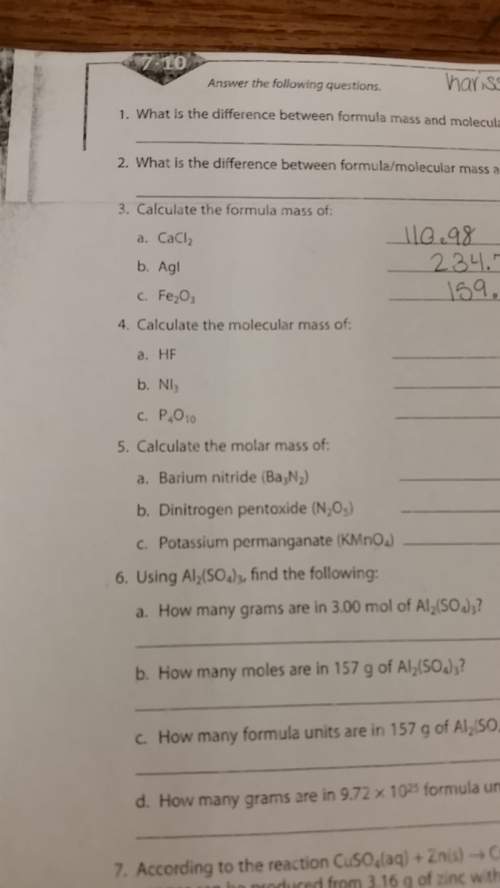

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1


Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
4. calculate the molecular mass of:
a. hf
b. ni3
c. p4o10
...
a. hf
b. ni3
c. p4o10
...
Questions in other subjects:


Mathematics, 02.04.2021 21:00

Mathematics, 02.04.2021 21:00

Mathematics, 02.04.2021 21:00








