
Chemistry, 25.07.2019 14:00 ecoagro1934
A52.0-ml volume of 0.35 m ch3cooh (ka=1.8×10−5) is titrated with 0.40 m naoh. calculate the ph after the addition of 27.0 ml of naoh. express your answer numerically.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3



Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
A52.0-ml volume of 0.35 m ch3cooh (ka=1.8×10−5) is titrated with 0.40 m naoh. calculate the ph after...
Questions in other subjects:




Mathematics, 24.02.2021 16:00


History, 24.02.2021 16:00


Mathematics, 24.02.2021 16:00

Biology, 24.02.2021 16:00

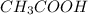 and
and  .
.
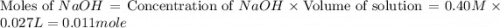

![[H^+]=\frac{\text{Moles of }H^+}{\text{Total volume}}](/tpl/images/0131/3396/b6338.png)
![[H^+]=\frac{0.0007mole}{(52+27)mL}=8.9\times 10^{-6}mole/mL=8.9\times 10^{-3}M](/tpl/images/0131/3396/73338.png)
![pH=-\log [H^+]](/tpl/images/0131/3396/37e81.png)




