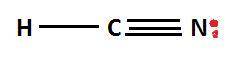Chemistry, 26.07.2019 04:00 melissa3333
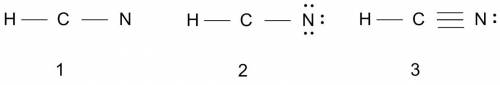
What is the lewis structure of the covalent compound that contains one nitrogen atom, one hydrogen atom, and one carbon atom? to add lone pairs, be sure to click the button lone-pair button before clicking on the molecule to add the lone pairs. use a line to connect each atom and indicate a bond. draw the molecule by placing atoms on the grid and connecting them with bonds. include all lone pairs of electrons?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

You know the right answer?
What is the lewis structure of the covalent compound that contains one nitrogen atom, one hydrogen a...
Questions in other subjects:



History, 25.05.2021 19:40