
Chemistry, 26.12.2019 09:31 CrusaderLord
Calculate the ph for each of the following cases in the titration of 50.0 ml of 0.210 m hclo(aq) with 0.210 m koh(aq).
(a) before addition of any koh
(b) after addition of 25.0 ml of koh
(c) after addition of 35.0 ml of koh
(d) after addition of 50.0 ml of koh
(e) after addition of 60.0 ml of koh

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1


Chemistry, 22.06.2019 22:30, lori90
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 23.06.2019 02:00, Turtlelover05
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3
You know the right answer?
Calculate the ph for each of the following cases in the titration of 50.0 ml of 0.210 m hclo(aq) wit...
Questions in other subjects:


Mathematics, 04.10.2021 07:40

Mathematics, 04.10.2021 07:40

Mathematics, 04.10.2021 07:40

History, 04.10.2021 07:40


English, 04.10.2021 07:40


Mathematics, 04.10.2021 07:40

Mathematics, 04.10.2021 07:40

![\text}{K}_{\text{a}} =\dfrac{ [H^{+}]^{2}}{\text{[HClO]}}](/tpl/images/0433/6132/82a3e.png)
![4 \times 10^{-8} =\dfrac{ [H^{+}]^{2}}{\text{[0.21]}}](/tpl/images/0433/6132/b84d3.png)
![[H^{+}]^{2} =4 \times 10^{-8} \times \text{0.21}](/tpl/images/0433/6132/f815a.png)
![[H^{+}]^{2} &= 8.4 \times 10^{-9 }](/tpl/images/0433/6132/4a2dc.png)
![[H^{+}] &= \sqrt{8.4 \times 10^{-9 }}](/tpl/images/0433/6132/0de98.png)
![[H^{+}] = 9.2 \times 10^{-5} \text{M}](/tpl/images/0433/6132/61a37.png)
![\text{pH} = - \text{log} [H^{+}]](/tpl/images/0433/6132/6fe5d.png)
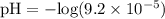
![\rm pH = Pka + log\dfrac{[Salt]}{[Acid]}](/tpl/images/0433/6132/d0693.png)
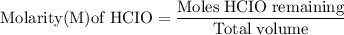
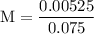
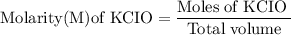
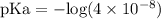
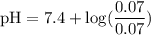
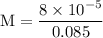
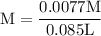
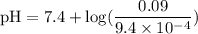
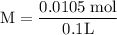
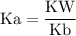
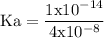
![\rm Kb = \dfrac {[CIO^{-}] [OH^{-}] }{ [KCIO]}](/tpl/images/0433/6132/0eaa1.png)
![\rm Kb = \dfrac {[OH^{-}] ^{2}}{ [KCIO]}](/tpl/images/0433/6132/2e104.png)
![\rm 2.5 x 10^{-7}= \dfrac {[OH^{-}] ^{2}}{ [0.105]}](/tpl/images/0433/6132/9ef1e.png)
![\rm pOH = - log [OH^{-}]](/tpl/images/0433/6132/91480.png)


