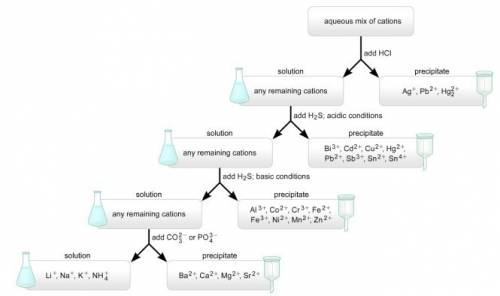
Asolution containing a mixture of metal cations was treated with dilute hcl and no precipitate formed. next, h2s was bubbled through the acidic solution. a precipitate formed and was filtered off. then, the ph was raised to about 8 and h2s was again bubbled through the solution. a precipitate again formed and was filtered off. finally, the solution was treated with a sodium carbonate solution, which resulted in no precipitation. which metal ions were definitely present, which were definitely absent, and which may or may not have been present in the original mixture?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 17:00, BREBRE8932
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3


Chemistry, 23.06.2019 02:30, babbity2009
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
Asolution containing a mixture of metal cations was treated with dilute hcl and no precipitate forme...
Questions in other subjects:

History, 04.08.2019 15:30






History, 04.08.2019 15:30


Biology, 04.08.2019 15:30

Mathematics, 04.08.2019 15:30