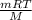Many gases are shipped in high-pressure containers. consider a steel tank whose volume is 55.0 gallons that contains o2 gas at a pressure of 16,500 kpa at 23 °c. (a) what mass of o2 does the tank contain? (b) what volume would the gas occupy at stp? (c) at what temperature would the pressure in the tank equal 150.0 atm? (d) what would be the pressure of the gas, in kpa, if it were transferred to a container at 24 °c whose volume is 55.0 l?

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:30, tateandvioletAHS14AY
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 00:40, joe7977
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
Many gases are shipped in high-pressure containers. consider a steel tank whose volume is 55.0 gallo...
Questions in other subjects:

Biology, 17.12.2020 02:00

History, 17.12.2020 02:00

English, 17.12.2020 02:00

Physics, 17.12.2020 02:00

Mathematics, 17.12.2020 02:00



Mathematics, 17.12.2020 02:00

Mathematics, 17.12.2020 02:00