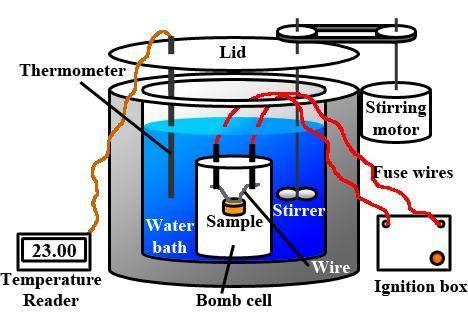
Assume your calorimeter is not perfect. burning 0.51 g of ethanol resulted in a 10 degree c rise in the temperature of the 200 ml of water in your calorimeter. how much energy was released by burning 0.51 g of ethanol? select one: a. 1386.1 kj b. 0.15 kj c. 29.4 kj d. 15.2 kj

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 08:00, gomezyonathan93
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1


Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Assume your calorimeter is not perfect. burning 0.51 g of ethanol resulted in a 10 degree c rise in...
Questions in other subjects:










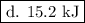 .
.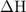 .
.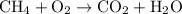
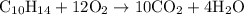
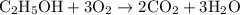
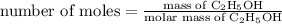 …… (1)
…… (1)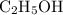 is 0.15 g.
is 0.15 g.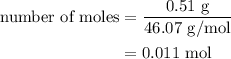
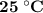 is
is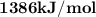 .
.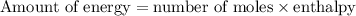 …… (2)
…… (2)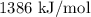 for enthalpy change in equation (2)
for enthalpy change in equation (2)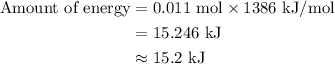
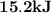 .
.