Answers: 1


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 00:00, chloe8979
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
What, if any, precipitate forms if aqueous solutions of lead(ii) nitrate (pb(no3)2) and sodium aceta...
Questions in other subjects:

Mathematics, 24.06.2021 16:10


Mathematics, 24.06.2021 16:10



Social Studies, 24.06.2021 16:10

Mathematics, 24.06.2021 16:10



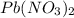 and
and 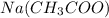 forms
forms 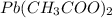 and
and 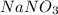 .
.