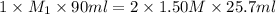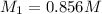Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:00, shradhwaip2426
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 23:00, tovarclaudia055
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
You know the right answer?
Avolume of 90.0 ml of aqueous potassium hydroxide (koh) was titrated against a standard solution of...
Questions in other subjects:

Mathematics, 21.06.2019 22:00




Chemistry, 21.06.2019 22:00

Chemistry, 21.06.2019 22:00


History, 21.06.2019 22:00


History, 21.06.2019 22:00

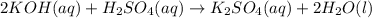
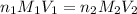
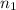 = acidity of an base = 1
= acidity of an base = 1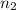 = basicity of an acid = 2
= basicity of an acid = 2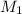 = concentration of
= concentration of 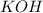 = ?
= ?
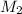 = concentration of
= concentration of 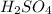 = 1.50 M
= 1.50 M
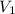 = volume of
= volume of 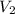 = volume of
= volume of