
Chemistry, 26.07.2019 15:30 CadenSkinner2003
A0.100 l solution of 0.300 m agno3 is combined with a 0.100 l solution of 1.00 m na3po4. calculate the concentration of ag and po43– at equilibrium after the precipitation of ag3po4 (ksp = 8.89 × 10–17).

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:30, hellokitty1647
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i. e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2
You know the right answer?
A0.100 l solution of 0.300 m agno3 is combined with a 0.100 l solution of 1.00 m na3po4. calculate t...
Questions in other subjects:

History, 27.03.2020 02:54




Biology, 27.03.2020 02:54

History, 27.03.2020 02:54

Biology, 27.03.2020 02:54

English, 27.03.2020 02:54


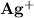 is 0.02998 M while the concentration of
is 0.02998 M while the concentration of 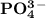 is 0.0999 M.
is 0.0999 M.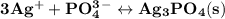
![\rm \bold{ Ksp = [ Ag^+]^3[ PO_4^3^- ]}](/tpl/images/0135/4274/43c55.png)
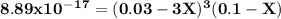
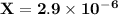
![\rm \bold{ [ Ag^+] = 0.02998 M}\\\\\\rm \bold{ [PO_4^3^-] = 0.0999 M }](/tpl/images/0135/4274/20a20.png)


