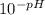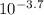Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, dpazmembreno
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2
You know the right answer?
If the ph of a 1.00-in. rainfall over 1400 miles2 is 3.70, how many kilograms of sulfuric acid, h2so...
Questions in other subjects:



Mathematics, 11.11.2020 02:00

Chemistry, 11.11.2020 02:00


Mathematics, 11.11.2020 02:00

Mathematics, 11.11.2020 02:00



Biology, 11.11.2020 02:00