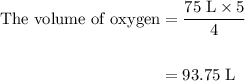Acetylene gas (c2h2) reacts with oxygen gas (o2) to produce carbon dioxide (co2) and water vapor (h2o). how many liters of c2h2 are required to produce 75.0 l of co2? l what volume of h2o is produced? l what volume of o2 is required? l when making the calculations, did you need to find the number of moles?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, boonkgang6821
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1


Chemistry, 22.06.2019 07:00, ceeejay0621
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1
You know the right answer?
Acetylene gas (c2h2) reacts with oxygen gas (o2) to produce carbon dioxide (co2) and water vapor (h2...
Questions in other subjects:


English, 24.08.2019 22:00

Mathematics, 24.08.2019 22:00


English, 24.08.2019 22:10

Chemistry, 24.08.2019 22:10


Social Studies, 24.08.2019 22:10

Mathematics, 24.08.2019 22:10

Mathematics, 24.08.2019 22:10

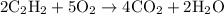
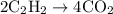
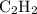 = 4 L of
= 4 L of 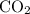
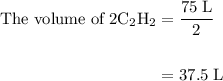
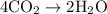
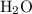
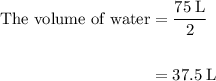
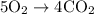
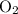 = 4 L of
= 4 L of