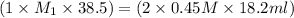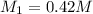If it requires 18.2 milliliters of 0.45 molar barium hydroxide to neutralize 38.5 milliliters of nitric acid, solve for the molarity of the nitric acid solution. show all of the work used to solve this problem. unbalanced equation: ba(oh)2 + hno3 yields ba(no3)2 + h2o

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2


Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
If it requires 18.2 milliliters of 0.45 molar barium hydroxide to neutralize 38.5 milliliters of nit...
Questions in other subjects:


English, 15.07.2019 15:00



Mathematics, 15.07.2019 15:00





Computers and Technology, 15.07.2019 15:00

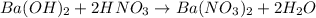
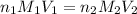
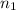 = basicity of
= basicity of 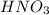 = 1
= 1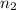 = acidity of
= acidity of 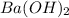 = 2
= 2
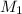 = concentration of
= concentration of 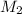 = concentration of
= concentration of 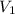 = volume of
= volume of 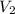 = volume of
= volume of