
Chemistry, 27.07.2019 05:30 timothymoles
The theoretical yield for a reaction is 55.9 g licl. the actual yield is 24.6 g licl. what is the percent yield of the reaction? question options: 227% 44% 25% 56%

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
The theoretical yield for a reaction is 55.9 g licl. the actual yield is 24.6 g licl. what is the pe...
Questions in other subjects:




Mathematics, 20.08.2019 23:50

History, 20.08.2019 23:50



History, 20.08.2019 23:50

Mathematics, 20.08.2019 23:50

History, 20.08.2019 23:50

 × 100
× 100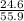 × 100
× 100

