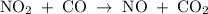The reaction between no2 and co to produce no and co2 is thought to occur in two steps: step 1: no2 + no2 no + no3 step 2: no3 + co no2 + co2 the experimental rate law is rate = k[no2]2. (a) write the equation for the overall reaction. do not include phase abbreviations. (b) identify the intermediate(s). no3 no2 co no co2

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:00, itasykamila
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 00:00, queenpaige2015
Which samples do the atoms have the least kinetic energy
Answers: 2
You know the right answer?
The reaction between no2 and co to produce no and co2 is thought to occur in two steps: step 1: no...
Questions in other subjects:



Mathematics, 18.01.2021 05:50

Physics, 18.01.2021 05:50



Mathematics, 18.01.2021 05:50

Mathematics, 18.01.2021 05:50

Mathematics, 18.01.2021 05:50

Mathematics, 18.01.2021 05:50

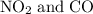 to produce NO and
to produce NO and 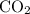 occurs in the given two steps.
occurs in the given two steps.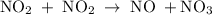

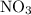 because it act as an intermediate and is utilized in the next step.
because it act as an intermediate and is utilized in the next step.