
Chemistry, 16.01.2020 05:31 skylerdemi1
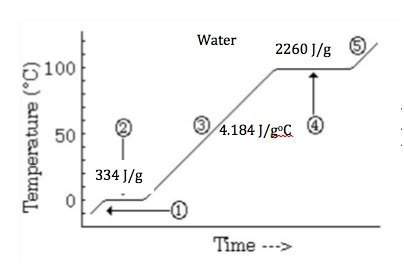
Elaborate the reason that the heat of vaporization of water is so much greater than its heat of fusion.
a) the potential energy of fusion represents a greater change of stored energy.
b) liquid water is stabilized by strong intermolecular forces between the molecules.
c) the kinetic energy of the water molecules is so much greater in the liquid state.
d) the bonding forces for solid water are much stronger than the bonding in the liquid.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2


Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Elaborate the reason that the heat of vaporization of water is so much greater than its heat of fusi...
Questions in other subjects:

Mathematics, 24.11.2019 22:31

History, 24.11.2019 23:31


Mathematics, 24.11.2019 23:31


Biology, 24.11.2019 23:31



English, 24.11.2019 23:31



