Chemistry, 27.07.2019 15:00 719563mercy
Gaseous butane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . what is the theoretical yield of water formed from the reaction of of 5.23 g butane and of 27.1 g oxygen gas? round your answer to significant figures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3
You know the right answer?
Gaseous butane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water ....
Questions in other subjects:


Mathematics, 04.02.2021 21:10





Mathematics, 04.02.2021 21:10

Chemistry, 04.02.2021 21:10

English, 04.02.2021 21:10

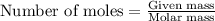 .....(1)
.....(1)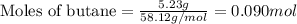
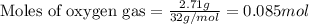
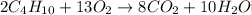
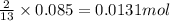 of butane
of butane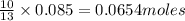 of water
of water