
Chemistry, 27.07.2019 19:00 sabree3940
Solid magnesium carbonate (mgco3) and solid sodium nitrate were mixed, placed in water, and stirred. what is observed? 1. mgco3 and nano3 will not dissolve. 2. mgco3 and nano3 will dissolve. 3. mgco3 will remain undissolved and nano3 will dissolve. 4. nano3 will remain undissolved and mgco3 will dissolve.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, marissastewart533
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2


Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
You know the right answer?
Solid magnesium carbonate (mgco3) and solid sodium nitrate were mixed, placed in water, and stirred....
Questions in other subjects:


English, 11.02.2020 10:12

History, 11.02.2020 10:12

Geography, 11.02.2020 10:12



History, 11.02.2020 10:12



Health, 11.02.2020 10:13

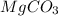 will remain undissolved and
will remain undissolved and 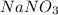 will dissolve.
will dissolve.

