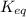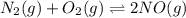Chemistry, 04.11.2019 11:31 janetshirinyan
Identify the proper form of the equilibrium-constant expression for the equation n2(g)+o2(g)⇌2no(g) view available hint(s) identify the proper form of the equilibrium-constant expression for the equation k=[no][n2][o2] k=[no]2[n2][o2] k=[n2][o2][no]2 k=2[no][n2][o2]

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, kakesheco4210
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3


You know the right answer?
Identify the proper form of the equilibrium-constant expression for the equation n2(g)+o2(g)⇌2no(g)...
Questions in other subjects:

Mathematics, 10.12.2020 21:20


Biology, 10.12.2020 21:20


Mathematics, 10.12.2020 21:20

Mathematics, 10.12.2020 21:20

Arts, 10.12.2020 21:20



![K_{eq}=\frac{[NO]^2}{[N_2][O_2]}](/tpl/images/0358/8006/3d926.png)