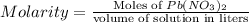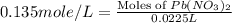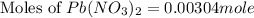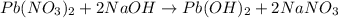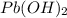Chemistry, 28.07.2019 01:00 cluchmasters5634
How many moles of lead (ii) hydroxide (solid) can be formed when 0.0225l of 0.135 m pb(no3)2 solution reacts with excess sodium hydroxide

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 23.06.2019 00:00, sanaiajohnson56
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 02:00, hayleebeals50
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
How many moles of lead (ii) hydroxide (solid) can be formed when 0.0225l of 0.135 m pb(no3)2 solutio...
Questions in other subjects:

Mathematics, 23.10.2019 06:00

Biology, 23.10.2019 06:00

Mathematics, 23.10.2019 06:00

Mathematics, 23.10.2019 06:00





Mathematics, 23.10.2019 06:00

Mathematics, 23.10.2019 06:00

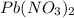 = 0.135 M = 0.135 mole/L
= 0.135 M = 0.135 mole/L