
Chemistry, 28.07.2019 04:00 aleyshamar14p95561
Co(g) + 2 h2 --> ch3oh 2.50 g of hydrogen is reacted with 30.0 l of carbon monoxide at stp. 1. what is the limiting reactant? *hint: only list the element symbol* 2. what mass of ch3oh is produced? *hint: only list the grams* 3. how much excess is left over? *hint: only list the grams*

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 18:30, Tyrant4life
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2


Chemistry, 23.06.2019 00:50, maddysmall32
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
Co(g) + 2 h2 --> ch3oh 2.50 g of hydrogen is reacted with 30.0 l of carbon monoxide at stp. 1. w...
Questions in other subjects:








Mathematics, 13.03.2020 17:33


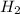
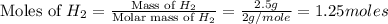
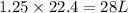 volume of hydrogen gas
volume of hydrogen gas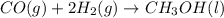
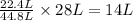 of carbon monoxide gas
of carbon monoxide gas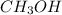 is, 20 grams
is, 20 grams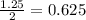 mole of
mole of 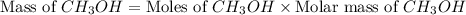
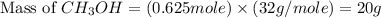
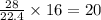 gram of carbon monoxide gas
gram of carbon monoxide gas

