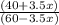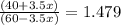Chemistry, 28.07.2019 07:00 kitkat033157
Suppose you have 1.00 l of an aqueous buffer containing 60.0 mmol acetic acid (pka = 4.76) and 40.0 mmol acetate. what volume of 3.50 m naoh would be required to increase the ph to 4.93?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, Vicky22Shz
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 1

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 20:30, ShahinF7536
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
Suppose you have 1.00 l of an aqueous buffer containing 60.0 mmol acetic acid (pka = 4.76) and 40.0...
Questions in other subjects:


Geography, 22.10.2020 19:01

Mathematics, 22.10.2020 19:01

Mathematics, 22.10.2020 19:01





Chemistry, 22.10.2020 19:01

![\frac{[CH_{3}COO^-]}{[CH_3COOH]}](/tpl/images/0141/9497/e74fb.png)