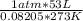Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, write2lakenor7awj
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 06:00, Chente379
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2
You know the right answer?
How many moles of o2 are in 53 l of o2 at stp? a) 2.4 b) 0.45 c) 1.65 d) 1187...
Questions in other subjects:



English, 16.04.2020 23:53

Mathematics, 16.04.2020 23:53

Mathematics, 16.04.2020 23:53

Mathematics, 16.04.2020 23:53

English, 16.04.2020 23:53



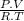 =
=