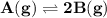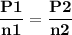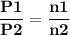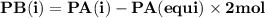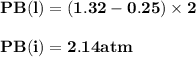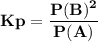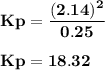Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, gracelanghorn
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1


Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
You know the right answer?
For the reaction a(g) 2 b(g), a reaction vessel initially contains only a at a pressure of pa = 1.32...
Questions in other subjects:





Mathematics, 05.09.2020 07:01


Chemistry, 05.09.2020 07:01


Mathematics, 05.09.2020 07:01