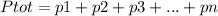Chemistry, 28.07.2019 17:00 laqu33n021
What does dalton's law of partial pressure say? a. the pressure contributed by larger gas molecules is more than smaller gases. b. the total pressure of a gas mixture is the sum of the individual pressures. c. the total pressures of a gas mixture is the average of the individual pressures. d. the pressure of a gas can only be partially known, since no gas is truly ideal

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2


Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
What does dalton's law of partial pressure say? a. the pressure contributed by larger gas molecules...
Questions in other subjects:


Mathematics, 12.09.2019 23:10

History, 12.09.2019 23:10


Mathematics, 12.09.2019 23:10


Mathematics, 12.09.2019 23:10


Health, 12.09.2019 23:10