Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1


Chemistry, 22.06.2019 11:00, RidhaH
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural. question 2 reflects a moral or social value. question 3 refers to something that can be measured. question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 14:00, MathChic68
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
The acid-dissociation constant for benzoic acid (c6h5cooh) is 6.3×10−5. calculate the equilibrium co...
Questions in other subjects:

Mathematics, 28.10.2019 21:31

History, 28.10.2019 21:31



Physics, 28.10.2019 21:31

History, 28.10.2019 21:31

Biology, 28.10.2019 21:31

Biology, 28.10.2019 21:31


![[C_6H_5COOH]_{eq}=0.06104M](/tpl/images/0143/6976/960a5.png)
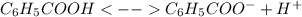
![Ka=\frac{[C_6H_5COO^-]_{eq}[H^+]_{eq}}{[C_6H_5COOH]_{eq}}](/tpl/images/0143/6976/5f347.png)
 , which alters the aforesaid equation in the following way, based on the ICE method:
, which alters the aforesaid equation in the following way, based on the ICE method:

![[C_6H_5COOH]_{eq}=0.0063M-0.00196M=0.06104M](/tpl/images/0143/6976/e3473.png)