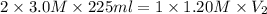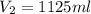Chemistry, 29.07.2019 01:30 kleathers97
Automobile batteries use 3.0 m h2so4 as an electrolyte. how much 1.20 m naoh will be needed to neutralize 225 ml of battery acid? h2so4(aq) + 2naoh(aq) --> 2h2o(l) + na2so4(aq)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, lukeakalucas
Alarge marble is dropped in a graduated cylinder with 35ml of water in it. the water level increases to 49ml. what is the volume of the marble
Answers: 1

You know the right answer?
Automobile batteries use 3.0 m h2so4 as an electrolyte. how much 1.20 m naoh will be needed to neutr...
Questions in other subjects:

Health, 30.06.2019 16:30


Health, 30.06.2019 16:30


Mathematics, 30.06.2019 16:30


Biology, 30.06.2019 16:30


Chemistry, 30.06.2019 16:30

 needed are, 1125 ml
needed are, 1125 ml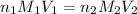
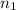 = basicity of an acid = 2
= basicity of an acid = 2
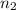 = acidity of a base = 1
= acidity of a base = 1
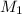 = concentration of
= concentration of 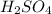 = 3.0 M
= 3.0 M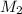 = concentration of NaOH = 1.20 M
= concentration of NaOH = 1.20 M
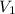 = volume of
= volume of 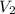 = volume of NaOH = ?
= volume of NaOH = ?