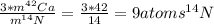Chemistry, 29.07.2019 11:00 CoolRahim9090
How many nitrogen-14 atoms does it take to equal the mass of three calcium-42 atoms? question 6 options: 8.5793impossible to determine without additional information?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 23.06.2019 01:20, cedricevans41p4j3kx
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1


Chemistry, 23.06.2019 04:40, twinchristiansp4xhd2
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
How many nitrogen-14 atoms does it take to equal the mass of three calcium-42 atoms? question 6 opti...
Questions in other subjects:



Mathematics, 04.07.2019 22:30

Mathematics, 04.07.2019 22:30


History, 04.07.2019 22:30


Mathematics, 04.07.2019 22:30