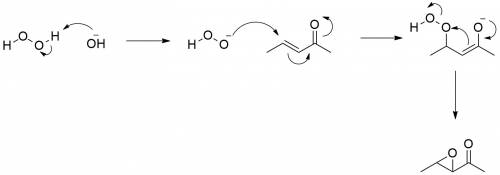
Treatment of an alpha, beta-unsaturated ketone with basic aqueous hydrogen peroxide yields an epoxy ketone. the reaction is specific to unsaturated ketones; isolated alkene double bonds do not react. on a sheet of paper, write a mechanism for the alkene to epoxide reaction. then, in the window below, draw the first intermediate in your mechanism.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:10, preachersgirl5
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1


Chemistry, 23.06.2019 18:30, choyontareq
You form water vapor by mixing oxygen and hydrogen at 730°c in a 5.4-liter container. this is the equation for the reaction: o2(g) + 2h2(g) → 2h2o(g). the partial pressure of oxygen before the reaction is 122.3 kilopascals, and there is excess hydrogen. how many moles of water are formed?
Answers: 3
You know the right answer?
Treatment of an alpha, beta-unsaturated ketone with basic aqueous hydrogen peroxide yields an epoxy...
Questions in other subjects:

Mathematics, 08.04.2021 18:50

Mathematics, 08.04.2021 18:50

Social Studies, 08.04.2021 18:50


Mathematics, 08.04.2021 18:50

Mathematics, 08.04.2021 18:50


Geography, 08.04.2021 18:50